Guidelines that can help diagnose iMCD
In order to diagnose a patient with idiopathic multicentric Castleman disease (iMCD), you must ensure they meet both Major Criteria and at least 2 of the 11 Minor Criteria, including ≥1 laboratory abnormality, and rule out diseases in the Exclusion Criteria.1
Major Criteria (need both)
Histology consistent with Castleman disease (CD)
≥2 enlarged lymph node groups
Minor Criteria (need ≥2, with ≥1 laboratory criterion)
Clinical abnormality
- Constitutional symptoms
- Large spleen and/or liver
- Fluid accumulation
- Violaceous papules
- Lymphocytic Interstitial pneumonitis
Laboratory abnormality
- Elevated C-reactive protein
- Anemia
- Thrombocytopenia
- Hypergammaglobulinemia
- Hypoalbuminemia
- Renal dysfunction
Exclusion Criteria

Exclude diseases that iMCD can mimic, such as autoimmune, malignant, and infectious diseases.
You can download a detailed, print-ready version of the Castleman Disease Collaborative Network diagnostic criteria, which includes a checklist that can help you during the diagnostic process.
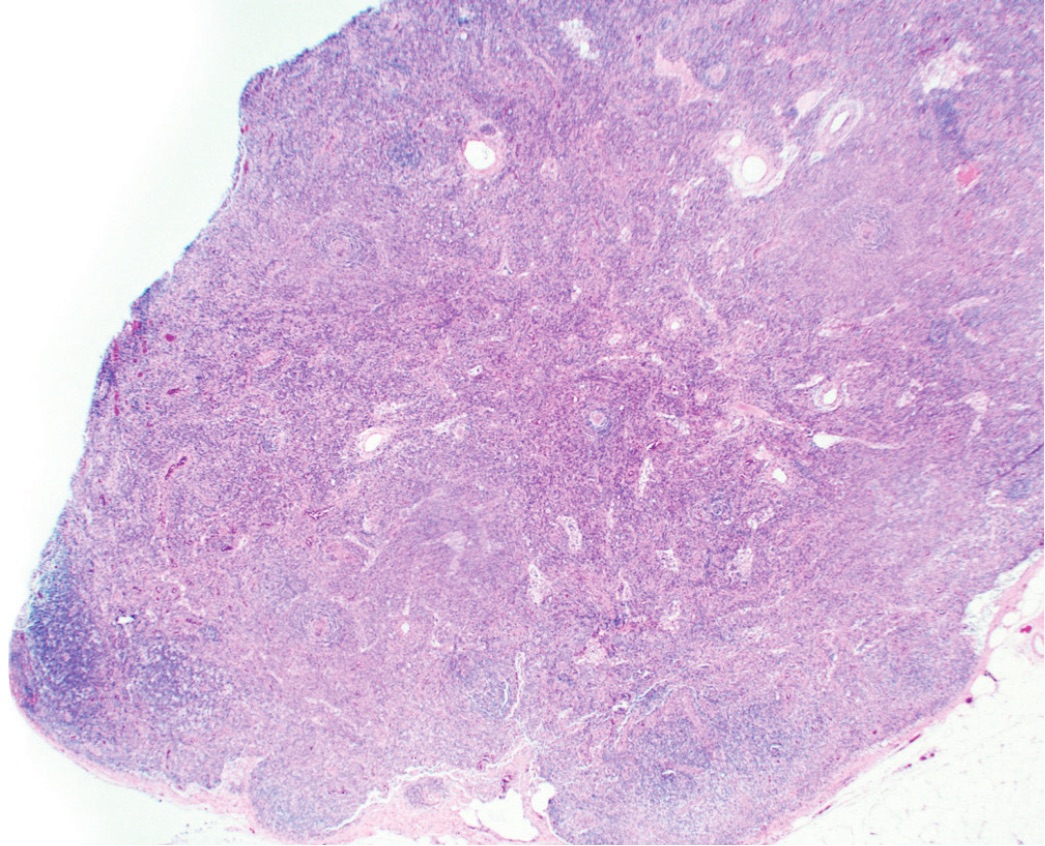

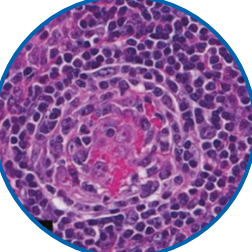


An excisional biopsy ensures pathologic confirmation
A lymph node biopsy is necessary to confirm the diagnosis of iMCD.1 There are two types of biopsies2:
Incisional (Core) Biopsy
Only extracts a small part of the enlarged lymph node
Excisional Biopsy
The enlarged lymph node is removed completely
The hallmarks of iMCD may be missed in an incisional biopsy,3 as shown in the image. The ideal way to diagnosis iMCD is with an excisional biopsy.4
The iMCD diagnostic journey may involve multiple specialists
For a patient who potentially has iMCD, the diagnostic journey may start at their primary care physician’s (PCP’s) office or at a hospital. Initially, doctors may suspect infectious, autoimmune, or malignant disorders, and patients may then be referred to other specialists.
If all other conditions have been ruled out, then doctors may perform an excisional biopsy, which may be the most effective way to diagnose this condition.1,4
Potential patient journey
This patient journey is not a complete representation of all the
doctors a patient may see in their journey to diagnosis.

A physician cannot diagnose iMCD without the help of a pathologist.1
References: 1. Fajgenbaum DC, Uldrick TS, Bagg A, et al. International, evidence-based consensus diagnostic criteria for HHV-8-negative/idiopathic multicentric Castleman disease. Blood. 2017;129(12):1646-1657. 2. Early detection, diagnosis, and staging of Castleman disease: tests for Castleman disease. American Cancer Society. https://www.cancer.org/content/dam/CRC/PDF/Public/8596.00.pdf. Revised February 2, 2018. Accessed August 15, 2022. 3. Allin D, David S, Jacob A, Mir N, Giles A, Gibbins N. Use of core biopsy in diagnosing cervical lymphadenopathy: a viable alternative to surgical excisional biopsy of lymph nodes? Ann R Coll Surg Engl. 2017;99(3):242-244. 4. Gaurin GE, da Costa Dourado CM. Castleman disease. Medscape. https://emedicine.medscape.com/article/2219018-overview. Updated November 24, 2021. Accessed August 15, 2022. 5. Infectious disease. American College of Physicians. https://www.acponline.org/about-acp/about-internal-medicine/subspecialties-of-internal-medicine/infectious-disease. Accessed August 15, 2022. 6. Rheumatologist. Dorland's Medical Dictionary Online. https://www.dorlandsonline.com/dorland/definition?id=43710. Accessed August 15, 2022. 7. Pathology. Dorland's Medical Dictionary Online. https://www.dorlandsonline.com/dorland/definition?id=37219. Accessed August 15, 2022. 8. Pathologist. Dorland's Medical Dictionary Online. https://www.dorlandsonline.com/dorland/definition?id=37218&searchterm=pathologist. Accessed August 15, 2022.